Association of Biologic Prescribing for Inflammatory Bowel Disease With Industry Payments to Physicians
Association of Biologic Prescribing for Inflammatory Bowel Disease With Industry Payments to Physicians
Abstract
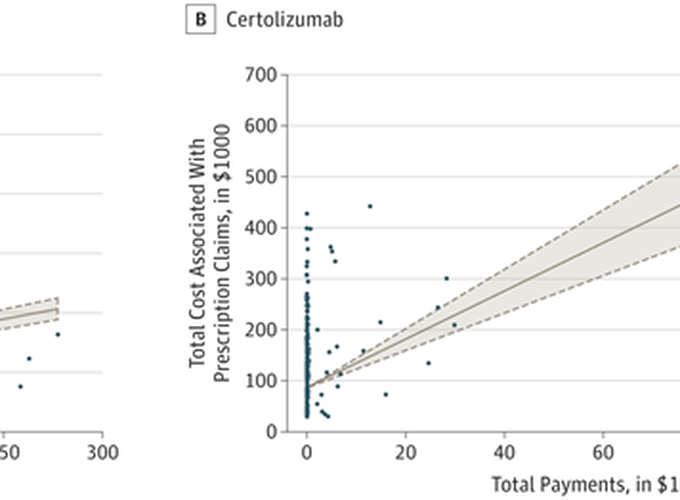
Biologic medications account for the majority of outpatient treatment expenditures for inflammatory bowel disease. The US Food and Drug Administration (FDA) approved adalimumab (Humira, AbbVie Inc) for Crohn disease (CD) in 2007 and ulcerative colitis (UC) in 2012 and approved certolizumab (Cimzia, Union Chimique Belge) for CD in 2006. For Medicare beneficiaries, adalimumab and certolizumab are the biologic agents prescribed by the largest number of gastroenterologists. These gastroenterologists frequently receive payments from the manufacturers of high-revenue medications. We investigated the association between payments from the manufacturers of these drugs to gastroenterologists and Medicare spending on these drugs.
Type
Publication
JAMA Internal Medicine